Abstract
The large nose adorned by adult male proboscis monkeys is hypothesised to serve as an audiovisual signal of sexual selection. It serves as a visual signal of male quality and social status, and as an acoustic signal, through the expression of loud, low-formant nasalised calls in dense rainforests, where visibility is poor. However, it is unclear how the male proboscis monkey nasal complex, including the internal structure of the nose, plays a role in visual or acoustic signalling. Here, we use cranionasal data to assess whether large noses found in male proboscis monkeys serve visual and/or acoustic signalling functions. Our findings support a visual signalling function for male nasal enlargement through a relatively high degree of nasal aperture sexual size dimorphism, the craniofacial region to which nasal soft tissue attaches. We additionally find nasal aperture size increases beyond dental maturity among male proboscis monkeys, consistent with the visual signalling hypothesis. We show that the cranionasal region has an acoustic signalling role through pronounced nasal cavity sexual shape dimorphism, wherein male nasal cavity shape allows the expression of loud, low-formant nasalised calls. Our findings provide robust support for the male proboscis monkey nasal complex serving both visual and acoustic functions.
Similar content being viewed by others
Introduction
Among many mammals, exaggerated sexually-selected facial traits in males serve as honest signals of fighting ability, and influence female mate choice1,2,3. Among primates, the large, bulbous nose of male adult proboscis monkeys (Nasalis larvatus) is unique, and has long been recognised as a sexually dimorphic trait, whose emergence has been linked to sexual selection through audiovisual signalling3,4,5,6,7,8,9,10,11. It serves as a visual signal, where male nasal enlargement is associated with the number of adult females per proboscis monkey breeding group, and the noses of males who are in breeding groups are significantly larger than those of males in non-breeding, all male groups10. There is evidence that enlarged nasal structures among male proboscis monkeys also play a role in acoustic communication, where larger formant ratios (a measure of vocal resonance properties) are tentatively associated with larger male nose size10. Therefore, enlarged nasal structures in male proboscis monkeys may allow them to emit louder or deeper nasal vocalisations, namely brays, honks and nasal roars, which communicate dominance and aggression, often in the context of intermale competition, intergroup communication and group protection12,13,14,15. This allows male proboscis monkeys with enlarged nasal structures to enhance their reproductive success.
The extent to which enlarged nasal structures in male proboscis monkeys evolved due to selective pressures associated with visual signalling or acoustic signalling, respectively, is difficult to ascertain by examining the nasal fleshy soft tissue alone10. However, understanding variation associated with male proboscis monkey cranionasal morphology, i.e. the nasal cavity (the vaulted chamber representing the bony cavity and the nasopharynx of the most superior and anterior part of the respiratory tract)16,17, including the nasal aperture (the bony anterior limit of the nasal cavity)16 may provide important insights into how and why enlarged nasal structures evolved in male proboscis monkeys, in association with audiovisual signalling. In the context of visual signalling, the nasal aperture is the bony region to which nasal soft tissue attaches18. This cranial attachment area therefore serves as a bony correlate of the nasal soft tissue morphology exhibited by male and female proboscis monkeys. Turning to acoustic signalling, male proboscis monkeys elicit two types of vocalisations that heavily rely on the nasal region of the vocal tract (herein referred to as ‘nasalised vocalisations’): honks and nasal roars, that are rarely elicited by females, if at all6,12,14,19. Male proboscis monkeys also elicit brays, a third type of nasalised vocalisation10, more frequently than do females15. Honks and nasal roars are characterised as loud calls based on their low fundamental frequencies (honks: 140 Hz; nasal roars: 137 Hz), and the high concentration of energy with which they are emitted14. Brays have fundamental frequencies of 85 Hz and are also characterised as loud calls10,14. By contrast, shrieks, which are the main vocalisation type expressed by females and immature proboscis monkeys15, are scream-like, higher fundamental frequency vocalisations (1350 Hz), are not considered to be loud calls, and do not heavily rely on the nasal region of the vocal tract14,20. As brays, honks and nasal roars are emitted by adult male proboscis monkeys using the nasal vocal tract6,10,12,14,15,19, if enlarged nasal structures are associated with acoustic communication, selection for increased nasal cavity size is expected among male proboscis monkeys, relative to females and other cercopithecoid taxa who do not elicit high amplitude nasalised loud calls. Further, the nasal cavity among male proboscis monkeys is expected to show a unique shape, capable of emitting loud and deep (high-amplitude, low formant) nasalised vocalisations.
Morphological traits that are under sexual selection associated with courtship and aggressive displays are expected to show positive static allometry because relatively large traits are an honest signal of an individual’s ability to win competitive encounters, and occur when the fitness gains of possessing a given trait are greater among larger animals than among smaller animals21,22. Among proboscis monkeys, if the nasal aperture or nasal cavity serves as a visual or acoustic signal of body size or fertility (i.e. through testis size)10, positive allometry in nasal aperture or cavity size is expected, depending on the function of nasal enlargement as an audiovisual trait. Sexual selection can additionally be detected using cranial variables based on assessments of the timing of maturity of the morphological trait under scrutiny, in the context of the timing of social maturity of the species being considered. For example, sagittal crest size among gorilla males, as measured using head profiles using digital photogrammetric data, is positively associated with the number of adult females per adult male, higher male offspring siring rates and offspring survival rates23,24. Male sagittal crest size, in conjunction with back breadth, is also associated with dominance rank, alpha male tenure length and the frequency of aggression25. Consistent with these behavioural data, the male gorilla sagittal crest shows positive static allometry when scaled against cranial size, and the timing of sagittal crest emergence among gorilla males in early adulthood coincides with the timing of social maturity26. Among proboscis monkeys, only adult males with large, developed noses live in uni-male, multi-female breeding groups, though younger males with smaller noses live exclusively in all-male groups5,10,27,28. These findings are consistent with evidence that male proboscis monkey large bulbous noses are a sexually selected trait, emerging in adulthood, and where older adult males have larger noses than their younger counterparts5,10,28,29. Therefore, if the male proboscis monkey nasal aperture or nasal cavity is associated with visual or acoustic signalling, size increases beyond dental maturity are expected.
As noted earlier, previous research has investigated soft tissue nasal morphology in the context of sexual selection through examining the relationships between relative nose size and body mass, testis size, number of adult females per breeding group, and vocal resonance properties10. However, no research has yet investigated enlarged nasal structures in male proboscis monkeys in the context of visual and acoustic signalling, using cranionasal data. In this study, we evaluate whether there is support for the ‘visual signalling hypothesis’ (the hypothesis that enlarged nasal structures evolved as a visual signal of male quality) and the ‘acoustic signalling hypothesis’ (the hypothesis that enlarged nasal structures evolved to allow males to emit enhanced nasalised vocalisations) in male proboscis monkeys. We investigate whether proboscis monkey sexual size dimorphism of the nasal aperture, and sexual size and shape dimorphism of the nasal cavity, exceeds that of three other cercopithecoid species (Cercopithecus mitis, Colobus polykomos and Macaca fascicularis). We assess whether proboscis monkey cranionasal morphology is larger than expected for a given cranial size by examining the allometric relationships between nasal aperture size, nasal cavity size and cranial size among male and female proboscis monkeys. Finally, we assess nasal aperture and nasal cavity size changes with age beyond dental maturity. We outline our predictions under the ‘visual signalling hypothesis’ and the ‘acoustic signalling hypothesis’ for each analysis in Table 1. The visual signalling hypothesis and the acoustic signalling hypothesis are not mutually exclusive, as large noses among male proboscis monkeys could simultaneously function as both a visual and as an acoustic signal.
Results
Sexual size dimorphism
The nasal aperture of male proboscis monkeys is 29% larger than that of females (Table 2). This relationship remains significant after controlling for body mass (t(31) = 2.550, p = 0.016). The degree of nasal aperture sexual size dimorphism in proboscis monkeys exceeds that of the other three cercopithecoid species, where among these species, male nasal aperture size is 7–15% larger than that of females (Table 2).
The nasal cavity of male proboscis monkeys is 26% larger than that of females (Table 2). This relationship also remains significant after correcting for body size (t(30) = 3.137, p = 0.004). Similar to what is observed for the nasal aperture, nasal cavity sexual size dimorphism in proboscis monkeys exceeds that of the other three cercopithecoid species, where among these species, male nasal cavity size is 7–17% larger than that of females (Table 2).
Sexual shape dimorphism
Proboscis monkeys show increased nasal cavity sexual shape dimorphism, relative to the three other cercopithecoid taxa (Table 3). Despite proboscis monkeys showing higher levels of body mass dimorphism than blue monkeys (C. mitis) and crab-eating macaques (M. fascicularis)30,31 (Supplementary Table 1), the percentage of nasal cavity shape variation accounted for by size in proboscis monkeys is lower than that of these two species (Table 3). This indicates that the high level of nasal cavity sexual shape dimorphism found in proboscis monkeys is not an artifact of size.
Visualisations of proboscis monkeys male and female nasal cavity shapes, relative to the mean proboscis monkey nasal cavity shape, show that there are sex differences in the superior and inferior nasal margins, and in the relative position of the superior lacrimal fossa (Fig. 1). Shape comparisons of the superior and inferior nasal margins show that the lateral aspect of the superior nasal margin among male proboscis monkeys (LM7) is more superiorly placed, relative to females, and the lateral aspect of the inferior nasal margin among males (LM9) is more inferiorly and laterally placed, relative to females (Fig. 1). However, the relative width of the nose at the widest point (LM8) is similar in both sexes, with females displaying a relatively wide superior nasal aperture (Fig. 1). Therefore, there are sex differences in the relative shape of the nasal aperture opening, where the nasal aperture is more ‘eggplant’ shaped in males, whilst resembling an ‘upside down pear’ in females (Fig. 1). The superior lacrimal fossa (LM6) is posteriorly positioned in males, relative to females, resulting in a relatively longer and lower nasal chamber in lateral aspect among males (Fig. 1). There are no substantial sex differences in the relative position of rhinion, nasospinale and alare; nor are there substantial differences between males and females in the posterior landmarks of the nasal cavity (Fig. 1). We consider how these nasal cavity shape differences are associated with acoustic signalling in the discussion section.
Wireframes showing mean nasal cavity shapes (Plots A,B) and mean nasal aperture shapes (Plots C,D) in proboscis monkeys. Plot (A) = mean male nasal cavity shape (blue), Plot (B) = mean female nasal cavity shape (red), Plot (C) = mean male nasal aperture shape (blue), Plot (D) = mean female nasal aperture shape (red). The wireframes shown in grey represent the mean nasal cavity and aperture shapes for males and females combined. Sex-specific nasal cavity and nasal aperture shapes have been scaled to a factor of 5 to more easily visualise which landmarks contribute to shape variation. Nasal cavity and nasal aperture landmarks are depicted in Supplementary Fig. S1, and landmark definitions are provided in Supplementary Table S2.
Allometry
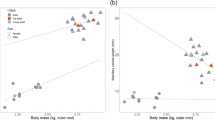
There is no significant relationship between log nasal aperture size and log cranial size among male or female proboscis monkeys (males: F(1,13) = 1.855, p = 0.196; females: F(1,9) = 0.128, p = 0.728), wherein among both males and females, nasal aperture size varies independently of cranial size. By contrast, the relationship between log nasal cavity size and log cranial size among male proboscis monkeys is significant (F(1,12) = 15.244, p = 0.002; Pearson’s r = 0.748, n = 14, p = 0.002; Fig. 2), wherein 52% of the variation in male nasal cavity size can be explained by cranial size. This relationship does not deviate from isometry (y = − 4.126 + 1.72x; 95% confidence interval: 0.761 < B < 2.683). The relationship between log nasal cavity size and log cranial size among female proboscis monkeys is not statistically significant (F(1,9) = 0.001, p = 0.971).
Log cranial size (x axis) vs log nasal cavity size (y axis) for proboscis monkeys. The blue triangles denote males and the red circles denote females. The male slope is statistically significant (F(1,12) = 15.244, p = 0.002). No female regression line is shown as there is no significant relationship between log cranial size and log nasal cavity size among females (F(1,9) = 0.001, p = 0.971).
Growth beyond dental maturity
Male proboscis monkeys show a positive, significant relationship between nasal aperture size and relative age (Spearman’s r = 0.453, n = 19, p = 0.026). No significant relationship between these two variables is found among female proboscis monkeys (Spearman’s r = 0.191, n = 14, p = 0.256). There is no significant relationship between nasal cavity size and relative age in male or female proboscis monkeys (males: Spearman’s r = 0.331, n = 18, p = 0.09; females: Spearman’s r = 0.007, n = 14, p = 0.491), nor is there a significant relationship between cranial size and relative age in either sex (males: Spearman’s r = 0.364, n = 15, p = 0.091; females: Spearman’s r = − 0.132, n = 13, p = 0.334).
Discussion
Our findings support both the visual and acoustic signalling hypotheses for enlarged nasal structures among male proboscis monkeys (Table 1). The visual signalling hypothesis is supported based on large nasal aperture size among males, i.e. the bony attachment area of the nasal soft tissue18, and male nasal aperture growth beyond dental maturity, consistent with enlarged nasal structures developing in mid-adulthood among some male proboscis monkeys5,10,27,28. The acoustic signalling hypothesis is supported based on increased nasal cavity sexual shape dimorphism among proboscis monkeys, and a longer, lower nasal cavity shape among males. Male proboscis monkey nasal cavity shape and enlarged nasal cavity size is consistent with the expression of low formant nasalised vocalisations. These include brays, honks and nasal roars, which are emitted at low fundamental frequencies (85–140 Hz) with a high concentration of energy, consistent with their categorisations as loud calls14. In particular, based on evidence that nasal and paranasal cavity morphology is associated with variation in formant frequency, in conjunction with overall vocal tract morphology32, the low, long and large nasal cavity observed in male proboscis monkeys found as part of the present study may facilitate the expression of the loud, low formant nasalised vocalisations observed among male proboscis monkeys6,12,14,15,19. High intensity primate vocalisations with low fundamental frequencies are associated with the condition of the individual and dominance status33,34 and therefore may be an honest indication of fighting ability, with lower fundamental frequency vocalisations preferred by females34. Our study findings further show sexual shape differences in the proboscis monkey nasal aperture, with an ‘eggplant’ shape in males, and an ‘upside down pear’ shape in females. Male nasal aperture shape therefore allows for greater concentration of sound to be passed through the nasal aperture, potentially allowing for high intensity sounds with low formants (increased resonance) to be emitted, consistent with the source-filter theory of vocalisations in non-human mammals as honest signals of male quality, body size or age35,36. The low formant frequency and the high amplitude of male proboscis monkey nasalised loud calls may be particularly important given that they live in forested environments, where the sounds expressed by animals need to propagate sufficiently to communicate information to individuals with whom they are not in visual proximity37,38.
Contrary to our expectations, in the present study we find no association between nasal aperture size and cranial size in either sex, though an isometric relationship between nasal cavity size and cranial size is observed among males. While our findings do not support either the visual signalling hypothesis or the acoustic signalling hypothesis under our study predictions, it is noteworthy that among male proboscis monkeys, nasal aperture size does not show a significant relationship with cranial size, while nasal cavity size does show this association. This indicates that nasal aperture size in male proboscis monkeys varies independently of nasal cavity size, consistent with the visual signalling hypothesis. Under this scenario, nasal aperture size variation is associated with the size of the fleshy nose and the timing of its enlargement, independent of cranial size. This interpretation is consistent with reports that nasal enhancement may be delayed in male proboscis monkeys until they have an opportunity to take over a breeding group29. There have been similar reports of the delayed adulthood onset of soft tissue secondary sexual characteristics among orangutans and capuchin monkeys39,40. Under this scenario, we suggest that if male proboscis monkey noses only increase when an opportunity to take over a breeding group presents itself, i.e. at different stages of adulthood based on when the opportunity arises, an association between nasal aperture size with cranial size may not expected, consistent with our study findings.
In the present study, we show that male proboscis monkey cranionasal morphology is associated with sexual selection, through both visual and acoustic signalling. The fleshy soft tissue of the nasal apparatus has previously been associated with audiovisual signalling among male proboscis monkeys10 and it is therefore likely that internal nasal cavity size and shape work in conjunction with the enhanced fleshy nose tissue to emit the unique nasalised vocalisations found in this species. This is in addition to the role of the nasal soft tissue, and its bony attachment area correlates, in visual signalling. Behavioural data support the interpretation that the nasal soft tissue and nasal cavity work together to emit unique proboscis monkey nasalised vocalisations, where during nasal honks, male proboscis monkeys reportedly rigidly straighten their noses in an upwards direction with each honk19. A similar phenomenon has been observed in male saigas, who too have prominent nasal morphology, and who tense and extend their noses when emitting nasal roars41. Therefore, enlarged nasal morphology in the context of audiovisual signalling among proboscis monkeys, while unique among primates, has been observed in other mammals. The results of our study build on previous evidence that primate craniofacial regions can carry a signal of sexual selection26,42,43 and our findings have implications in their potential applications for interpreting extinct primate social behaviour.
Methods
Study sample and 3D model acquisition
The sample consists of 142 dentally mature cranial specimens belonging to four cercopithecoid species (C. mitis, C. polykomos, M. fasciluaris, N. larvatus) (Supplementary Table S1). The species of interest is N. larvatus, and we selected the three other cercopithecoid species as a comparative sample to compare N. larvatus cranionasal sexual size and shape dimorphism. We selected C. polykomos as a second colobine species that is closely related to N. larvatus, and selected M. fascicularis and C. mitis as more distantly related cercopithecoid species that show moderate-high levels of body mass dimorphism, approaching the level observed in N. larvatus31 (Supplementary Table S1). None of the three comparative taxa (C. mitis, C. polykomos, M. fasciluaris) use nasalised loud calls as part of their modes of auditory communication. 3D models were obtained using a Polyga HDI Advance RX4 or a LMI Technologies HDI 3D scanner. Differences in the type of scanner used are unlikely to introduce substantial measurement error based on the specimen size of the species sampled as part of this study44.
Morphometric size and shape variables
We quantified nasal aperture and nasal cavity size and shape using 3D landmarks, adapted from Noback et al.17, taken from 3D surface models using Checkpoint v. 2022.12.16.0419 (Supplementary Fig. S1; Supplementary Table S2). We quantified nasal aperture size as the geometric mean of three linear measurements, and quantified nasal cavity size as the geometric mean of seven linear measurements (Supplementary Table S3).
We estimated body mass as follows: orbital breadth (the minimum distance between maxillofrontale and ectoconchion) × orbital height (the minimum distance between upper and lower margins of the orbital cavity, taken at a right angle to orbital breadth) × π, based on evidence that orbital area as an ellipse is a strong predictor of body mass45. We quantified cranial size as the geometric mean of the following three measurements: (1) cranial height: the distance between superior cranial vault (the point the sagittal plane of the cranial vault that intersects with the posterior limits of the zygomatic process in Frankfurt horizontal) and basion (the anterior-most point of the foramen magnum), (2) cranial length: the distance between glabella (the most anterior point between the orbits at the midline) and opisthocranion (the most posterior point of the cranium at the Frankfurt horizontal at the midline), and (3) cranial breadth: the distance between the left and right midpoints of the zygomatic arch at the widest point.
We quantified nasal cavity shape by performing generalised Procrustes analysis (GPA) on 12 3D landmarks (Supplementary Fig. S1; Supplementary Table S2) for all four species combined. 3D landmarks were scaled to a standard centroid size, then translated and rotated to minimise the squared distances between sets of landmarks46.
Estimating relative adult age
To assess relative adulthood age in N. larvatus specimens, we ranked specimens based on the degree of upper molar wear26,47 using screenshots of the dentition of each specimen, taken from 3D surface models. The youngest adult specimens show relatively low amounts of molar wear and the oldest specimens show relatively heavy molar wear. Anterior dentition and premolar wear were taken into account when there was not enough molar wear to adequately rank specimens. Males and females were ranked separately.
Statistical analysis
Sexual size dimorphism
We quantified nasal aperture and nasal cavity sexual size dimorphism by calculating the index of sexual size dimorphism (ISD), calculated as mean male size/mean female size42. We tested for statistically significant differences between mean male and mean female size values using Student’s t-tests. When performing t-tests, we used Levene’s test for homogeneity of variance. When the homogeneity of variance assumption was violated, we reported Welch’s test of equality of means. We performed sexual size dimorphism tests using SPSS v. 29.
Sexual shape dimorphism
We quantified nasal cavity sexual shape dimorphism by calculating the Procrustes Distance (PD) between the mean male nasal cavity shape and the mean female nasal cavity shape, using Procrustes shape coordinates from the GPA described earlier. We used permutation tests to assess whether the male-female PD was significant (10,000 permutations per analysis). We assessed the percentage of nasal cavity shape variation that was influenced by nasal cavity size using pooled within-group regression analyses and using permutation tests (10,000 permutations per analysis to assess statistical significance). We visualised N. larvatus nasal cavity shape using wireframes to compare the mean male, and mean female shape, respectively, with the mean N. larvatus shape. Shape visualisations were performed using Procrustes shape coordinates from GPAs, performed on the N. larvatus dataset. Mean male and mean female shape visualisations were performed using a scaling factor of five. We performed shape analyses and wireframe visualisations using MorphoJ v. 1.07a48.
Allometry
We assessed whether there were statistically significant relationships between nasal aperture size and nasal cavity size, respectively, and cranial size using ordinary least squared (OLS) regression on log transformed variables, using log cranial size as the dependent variable, and log nasal aperture or log nasal cavity size as the independent variable. We used ANOVA tests to assess model significance. When the model was significant we used Pearson’s correlation coefficient to assess the relationship between variables, and used the adjusted R2 value to assess how much nasal size variation is explained by cranial size variation. For models that were statistically significant, we tested for isometry by calculating the 95% confidence intervals of the regression value B, where B > 1 = positive allometry, B = 1 = isometry, and B < 1 = negative allometry.
Growth beyond dental maturity
We assessed whether there were nasal aperture, nasal cavity or cranial size increases with age beyond dental maturity using Spearman’s correlations between these size variables and tooth wear rank (1-tailed test).
Data availability
The dataset used in this study is available via Figshare: https://doi.org/10.6084/m9.figshare.24845043.
References
Darwin, C. The Descent of Man and Selection in Relation to Sex (John Murray, 1871).
Andersson, M. Sexual Selection (Princeton University Press, 1994).
Dixson, A., Dixson, B. & Anderson, M. Sexual selection and the evolution of visually conspicuous sexually dimorphic traits in Male Monkeys, Apes, and human beings. Annu. Rev. Sex Res. 16, 1–19. https://doi.org/10.1080/10532528.2005.10559826 (2005).
Schultz, A. H. Growth and development of the proboscis monkey. Bull. Mus. Comp. Zool. 89, 277–314 (1942).
Bennett, E. L. & Sebastian, A. C. Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. Int. J. Primatol. 9, 233–255. https://doi.org/10.1007/BF02737402 (1988).
Yeager, C. P. Proboscis monkey (Nasalis larvatus) social organization: Intergroup patterns of association. Am. J. Primatol. 23, 73–86. https://doi.org/10.1002/ajp.1350230202 (1991).
Ravosa, M. J. The ontogeny of cranial sexual dimorphism in two old world monkeys: Macaca fascicularis (Cercopithecinae) and Nasalis larvatus (Colobinae). Int. J. Primatol. 12, 403–426. https://doi.org/10.1007/BF02547620 (1991).
Murai, T. Mating behaviors of the proboscis monkey (Nasalis larvatus). Am. J. Primatol. 68, 832–837. https://doi.org/10.1002/ajp.20266 (2006).
Grueter, C. C. & van Schaik, C. P. Sexual size dimorphism in Asian colobines revisited. Am. J. Primatol. 71, 609–616. https://doi.org/10.1002/ajp.20695 (2009).
Koda, H. et al. Nasalization by Nasalis larvatus: Larger noses audiovisually advertise conspecifics in proboscis monkeys. Sci. Adv. 4, eaaq0250. https://doi.org/10.1126/sciadv.aaq0250 (2018).
Matsuda, I. et al. Large male proboscis monkeys have larger noses but smaller canines. Commun. Biol. 3, 522. https://doi.org/10.1038/s42003-020-01245-0 (2020).
Kawabe, M. & Mano, T. Ecology and behavior of the wild proboscis monkey, Nasalis larvatus (Wurmb), in Sabah, Malaysia. Primates 13, 213–227. https://doi.org/10.1007/BF01840882 (1972).
Yeager, C. P. Proboscis monkey (Nasalis larvatus) social organization: Nature and possible functions of intergroup patterns of association. Am. J. Primatol. 26, 133–137. https://doi.org/10.1002/ajp.1350260207 (1992).
Röper, K. M. et al. Vocal acoustics in the endangered proboscis monkey (Nasalis larvatus). Am. J. Primatol. 76, 192–201. https://doi.org/10.1002/ajp.22221 (2014).
Scheumann, M., Röper, K. M., Nathan, S. K. S. S. & Goossens, B. Third-party vocal intervention in the proboscis monkey (Nasalis larvatus). Int. J. Primatol. 43, 698–711. https://doi.org/10.1007/s10764-021-00273-9 (2022).
Mlynski, G., Grützenmacher, S., Plontke, S., Mlynski, B. & Lang, C. Correlation of nasal morphology and respiratory function. Rhinology 39, 197–201 (2001).
Noback, M. L., Harvati, K. & Spoor, F. Climate-related variation of the human nasal cavity. Am. J. Phys. Anthropol. 145, 599–614. https://doi.org/10.1002/ajpa.21523 (2011).
Bruintjes, T. D., Van Olphen, A. F., Hillen, B. & Huizing, E. H. A functional anatomic study of the relationship of the nasal cartilages and muscles to the nasal valve area. Laryngoscope 108, 1025–1032. https://doi.org/10.1097/00005537-199807000-00014 (1998).
Kern, J. A. Observations on the habits of the proboscis monkey, Nasalis larvatus (Wurmb), made in the Brunai Bay area, Borneo. Zoologica 49, 183–192 (1964).
Srivathsan, A. & Meier, R. Proboscis monkeys (Nasalis larvatus (Wurmb, 1787)) have unusually high-pitched vocalizations. Raffles Bull. Zool. 59, 319–323 (2011).
Bonduriansky, R. & Day, T. The evolution of static allometry in sexually selected traits. Evolution 57, 2450–2458. https://doi.org/10.1111/j.0014-3820.2003.tb01490.x (2003).
Eberhard, W. G. et al. Sexual selection and static allometry: The importance of function. Q. Rev. Biol. 93, 207–250. https://doi.org/10.1086/699410 (2018).
Caillaud, D., Levréro, F., Gatti, S., Ménard, N. & Raymond, M. Influence of male morphology on male mating status and behavior during interunit encounters in western lowland gorillas. Am. J. Phys. Anthropol. 135, 379–388. https://doi.org/10.1002/ajpa.20754 (2008).
Breuer, T., Robbins, A. M., Boesch, C. & Robbins, M. M. Phenotypic correlates of male reproductive success in western gorillas. J. Hum. Evol. 62, 466–472. https://doi.org/10.1016/j.jhevol.2012.01.006 (2012).
Wright, E. et al. Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim. Behav. 151, 87–102. https://doi.org/10.1016/j.anbehav.2019.03.011 (2019).
Balolia, K. L., Soligo, C. & Wood, B. Sagittal crest formation in great apes and gibbons. J. Anat. 230, 820–832. https://doi.org/10.1111/joa.12609 (2017).
Yeager, C. P. Proboscis monkey (Nasalis larvatus) social organization: Group structure. Am. J. Primatol. 20, 95–106. https://doi.org/10.1002/ajp.1350200204 (1990).
Murai, T. Social behaviors of all-male proboscis monkeys when joined by females. Ecol. Res. 19, 451–454. https://doi.org/10.1111/j.1440-1703.2004.00656.x (2004).
Hollihn, U. W. E. Remarks on the breeding and maintenance of Colobus monkeys Colobus guereza, Proboscis monkeys Nasalis larvatus and Douc langurs Pygathrix nemaeus in zoos. Int. Zoo Yearb. 13, 185–188. https://doi.org/10.1111/j.1748-1090.1973.tb02146.x (1973).
Smith, R. J. & Jungers, W. L. Body mass in comparative primatology. J. Hum. Evol. 32, 523–559. https://doi.org/10.1006/jhev.1996.0122 (1997).
Plavcan, J. M. Taxonomic variation in the patterns of craniofacial dimorphism in primates. J. Hum. Evol. 42, 579–608. https://doi.org/10.1006/jhev.2001.0542 (2002).
Dang, J., Honda, K. & Suzuki, H. Morphological and acoustical analysis of the nasal and the paranasal cavities. J. Acoust. Soc. Am. 96, 2088–2100. https://doi.org/10.1121/1.410150 (1994).
Fischer, J., Kitchen, D. M., Seyfarth, R. M. & Cheney, D. L. Baboon loud calls advertise male quality: Acoustic features and their relation to rank, age, and exhaustion. Behav. Ecol. Sociobiol. 56, 140–148. https://doi.org/10.1007/s00265-003-0739-4 (2004).
Delgado, R. A. Sexual selection in the loud calls of male primates: Signal content and function. Int. J. Primatol. 27, 5–25. https://doi.org/10.1007/s10764-005-9001-4 (2006).
Fitch, W.T., Hauser, M.D. Unpacking “Honesty”: vertebrate vocal production and the evolution of acoustic signals. In Acoustic Communication. Springer Handbook of Auditory Research, vol. 16 (eds. Simmons, A.M. et al.). (Springer, 2003). https://doi.org/10.1007/0-387-22762-8_3.
Taylor, A. M. & Reby, D. The contribution of source–filter theory to mammal vocal communication research. J. Zool. 280, 221–236. https://doi.org/10.1111/j.1469-7998.2009.00661.x (2010).
Richards, D. G. & Wiley, R. H. Reverberations and amplitude fluctuations in the propagation of sound in a forest: Implications for animal communication. Am. Nat. 115, 381–399. https://doi.org/10.1086/283568 (1980).
Mitani, J. C. & Stuht, J. The evolution of nonhuman primate loud calls: Acoustic adaptation for long-distance transmission. Primates 39, 171–182. https://doi.org/10.1007/BF02557729 (1998).
Utami, S. S., Goossens, B., Bruford, M. W., de Ruiter, J. R. & van Hooff, J. A. R. A. M. Male bimaturism and reproductive success in Sumatran orang-utans. Behav. Ecol. 13, 643–652 (2002). https://doi.org/10.1093/beheco/13.5.643.
Paukner, A., Slonecker, E. M. & Wooddell, L. J. Effects of dominance and female presence on secondary sexual characteristics in male tufted capuchin monkeys (Sapajus apella). Ecol. Evol. 11, 6315–6325. https://doi.org/10.1002/ece3.7483 (2021).
Frey, R., Volodin, I. & Volodina, E. A nose that roars: Anatomical specializations and behavioural features of rutting male saiga. J. Anat. 211, 717–736. https://doi.org/10.1111/j.1469-7580.2007.00818.x (2007).
Balolia, K. L. Craniodental sexual dimorphism among hylobatids. Int. J. Primatol. 42, 737–758. https://doi.org/10.1007/s10764-021-00233-3 (2021).
Fannin, L. D., Plavcan, J. M., Daegling, D. J. & McGraw, W. S. Oral processing, sexual selection, and size variation in the circumorbital region of Colobus and Piliocolobus. Am. J. Phys. Anthropol. 175, 559–576. https://doi.org/10.1002/ajpa.24280 (2021).
Balolia, K. L. & Massey, J. S. How does scanner choice and 3D model resolution affect data accuracy?. J. Anat. 238, 679–692. https://doi.org/10.1111/joa.13343 (2021).
Spocter, M. A. & Manger, P. R. The use of cranial variables for the estimation of body mass in fossil hominins. Am. J. Phys. Anthropol. 134, 92–105. https://doi.org/10.1002/ajpa.20641 (2007).
Rohlf, F. J. & Slice, D. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst. Biol. 39, 40–59. https://doi.org/10.2307/2992207 (1990).
Balolia, K. L., Soligo, C. & Lockwood, C. A. Sexual dimorphism and facial growth beyond dental maturity in great apes and gibbons. Int. J. Primatol. 34, 361–387. https://doi.org/10.1007/s10764-013-9666-z (2013).
Klingenberg, C. P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x (2011).
Acknowledgements
We thank the Terhune Lab, Department of Anthropology, University of Arkansas for making available 3D surface models of Nasalis larvatus, Colobus polykomos, Cercopithecus mitis and Macaca fascicularis, which were collected from the American Museum of Natural History, New York, the Smithsonian National Museum of Natural History, Washington DC, the Field Museum of Natural History, Chicago and the Royal Museum of Central Africa, Terverun. These 3D models were made available for research via MorphoSource.org. KLB would like to thank Alexander Rogers for helpful discussions on the physics of acoustics.
Author information
Authors and Affiliations
Contributions
Conceptualisation of the idea for the study: K.L.B.; Study development: P.L.F., K.L.B.; Data collection: K.L.B.; Statistical analysis: K.L.B.; Writing—original draft: K.L.B.; Writing—review & editing: K.L.B., P.L.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balolia, K.L., Fitzgerald, P.L. Male proboscis monkey cranionasal size and shape is associated with visual and acoustic signalling. Sci Rep 14, 10715 (2024). https://doi.org/10.1038/s41598-024-60665-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60665-8